Food drug and cosmetic act of 1938 pdf
Federal food, drug, and cosmetic act. Judicial and administrative record, 1938–1949. By Vincent A. Kleinfeld and Charles Wesley Dunn. Commerce Clearing House, Inc.,
PDF Plus ” Federal Food, Drug and Cosmetic Act, Judicial and Administrative Record, 1938-1949 .” American Journal of Public Health and the Nations Health , 40(7), pp. 885–886
THE FooD, DRuG, AND CosmETIc Aar oF 1938 3 provisions of the new law, indicating wherein they differ from those contained in the old law and in the original bill …
Food drug and cosmetic act 1938 1. BY: SWATI SARIN 2. Federal Food, Drug, and Cosmetic Act Acronym FFDCA, “FD&C Act” Enacted by the 75th United States Congress Citations Public Law 75-717 Stat. 52 US Stat. 1040 Codification U.S.C. sections created 21 U.S.C. § 301 et seq Legislative history • Signed into law by
The federal Food, Drug, and Cosmetic Act and other food and drug laws are codified in Title 21 of the United States Code. For information on accessing the United States Code and researching U.S. statutory law generally, see Georgetown Law Library’s Statutes Research Guide or the Statutory Research Tutorial.
The 1938 Federal Food, Drug, and Cosmetic Act (FD&C Act) took the first major step to remove these obstacles to regulation.4 Invention of “new drugs.” The most important change—requiring
1/01/2011 · Federal Food, Drug, and Cosmetic Act 1938 Katelyn Pollock and Forrest Averite. Federal Food, Drug, and Cosmetic Act The Federal Food, Drug, and Cosmetic Act was passed by Congress in 1938 as a national law and was amended in 1954 and 1958. This law deals with the pollution of our food, drugs, and cosmetics with harmful pesticides. Federal Food, Drug, and Cosmetic Act This …
6/10/2018 · The federal law spelled out in the decades-old Delaney Clause, which is an amendment to the Food, Drugs, and Cosmetic Act of 1938, stipulates that if …
Section 564 of the Federal Food, Drug, and Cosmetic Act Fact Sheet Overview. An Emergency Use Authorization (EUA) under Section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) 1 allows for the special use of drugs and other medical products during certain types of emergencies. 2 The EUA authority was added by the Project BioShield
21 U.S.C. 301 – Short title Publication Title: United States Code, 2006 Edition, Supplement 5, Title 21 – FOOD AND DRUGS
The Federal Food, Drug, and Cosmetic Act of 1938 (FD&C Act or the Act) promotes national public health by preventing fraudulent activity with respect to food, drugs, and an array of other public health products. 5 The FD&C Act and its implementing regulations contain standards to protect and promote public health, including requirements for prescription drug approval 6 and food safety. 7
It, therefore, might be time to question the goals of the Food, Drug and Cosmetic Act of 1938, enforcement policy, and whether the use of strict criminal liability is a necessary method of achieving those goals in the current setting. This paper attempts to analyze these questions by tracing the history of strict liability in the area of food and drug law violations, discussing the common
9/3/2010 1 The Federal Food, Drug, and Cosmetic Act of 1938, as Amended (21 USC, Chapter 9, Sec. 301-399a) Food, Drug, and Cosmetic Act •The major law related to food …
The month of July witnessed the beginning of enforcement of the Federal Food, Drug and Cosmetic Act of 1938. While the majority of the provisions of the act will not become effective until next June, dangerous drugs and dangerous cosmetics are immediately subject to the new law.
An Examination of Strict Criminal Liability Under the Food, Drug and Cosmetic Act of 1938: Is It Time For Change? Breena L. Holt October 18, 1999
AllergyEats: The leading guide to food allergy-friendly restaurants nationwide. Free app available in App Store and Google Play.
YouTube Embed: No video/playlist ID has been supplied
Federal Food Drug and Cosmetic Act 1938 Sustainability

Consumers Dictionary Of Cosmetic Ingredients
Act of June 25, 1938 (Federal Food, Drug, and Cosmetic Act), Public Law 75-717, 52 STAT 1040, which prohibited the movement in interstate commerce of adulterated and misbranded food, drugs, devices, and cosmetics, 6/25/1938
– Federal Food, Drug, and Cosmetic Act of 1938, as amended. To protect consumers from unsafe or deceptively labeled or packaged products by prohibiting the movement in interstate commerce of Sun, 16 Dec 2018 16:54:00 GMT Cosmetic Labeling Guide – Food and Drug Administration – Appendix I Annex I – Illustative List by Categories of Cosmetic Products ANNEX I ILLUSTRATIVE LIST BY
Eighty years ago on June 25, 1938, President Franklin D. Roosevelt signed the Federal Food, Drug, and Cosmetic Act. In recognition of this anniversary, we review how the FD&C Act came to be, how
Although the Federal Food, Drug, and Cosmetic Act defines various categories of food ingredients, it is frequently difficult to determine how to classify certain substances that meet the definitions of more than one category (Code of Federal Regulations, 1981). …
In the USA, these regulations are enforced by the U.S. Food and Drug Administration (FDA), as written in Section 501(B) of the Food, Drug, and Cosmetic act of 1938.
Food, Drug, and Cosmetic Act of 1938, as amended. To protect consumers from unsafe or deceptively labeled or packaged products by prohibiting the movement in interstate commerce of Mon, 31 Dec 2018 13:16:00 GMT Cosmetic Labeling Guide – Food and Drug Administration – BEEF HEART:. Although still fed to fish and often part of many homemade fish food recipes due to popular YouTube …
Administrative Law — Federal Food, Drug, and Cosmetic Act of 1938 — Laetrile and Other Drugs to Be Used by the Terminally Ill Are Not Exempt From the Safety and Effective Requirements of the Federal Food, Drug, and Cosmetic Act of 1938
For the purposes of the Federal Food, Drug, and Cosmetic Act of June 26, 1938, (ch. 675, sec. 1, 52 Stat. 1040) [21 U.S.C. 301 et seq.] nonfat dry milk is the product resulting from the removal of fat
Under the existing drug regulations, premarketing toxicity testing was not required. In reaction to this calamity, the U.S. Congress passed the 1938 Federal Food, Drug and Cosmetic Act, which required proof of safety before the release of a new drug. The 1938 law changed the drug focus of the Food and Drug Administration from that of a policing agency primarily concerned with the confiscation
The Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (Bioterrorism Act) is the greatest expansion in the Food and Drug Administration’s (FDA) food enforcement authorities since passage of the Federal Food, Drug, and Cosmetic Act (FD&C Act) in 1938.
A revision of the Pure Food and Drug Act, the “Federal Food Drug and Cosmetic Act,” passed in 1938, added several provisions that impacted the food industry. Among those provisions were authorized factory inspections and the authority for court injunction to the previous seizure and prosecution actions ( Janssen, 1992 ).
§301. Short title. This chapter may be cited as the Federal Food, Drug, and Cosmetic Act. (June 25, 1938, ch. 675, §1, 52 Stat. 1040.) Effective Date; Postponement

1938 TEnnessee drug company advertised a new sulfonamides elixir specifically intended for children. The toxic solvent was untested and more than 10 people died.
Today, more than 10 000 chemicals are allowed to be added to food and food contact materials in the United States, either directly or indirectly, under the 1958 Food Additives Amendment to the 1938 Federal Food, Drug, and Cosmetic Act (FFDCA) (public law number 85-929).
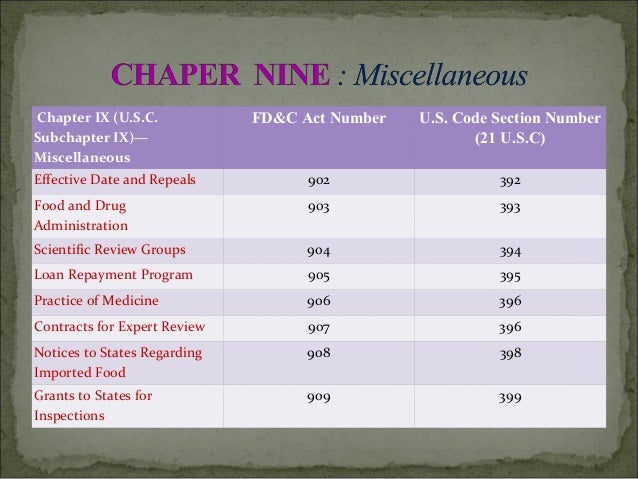
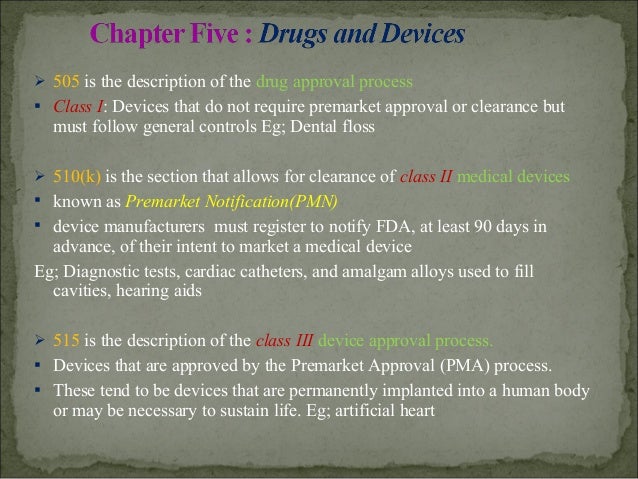
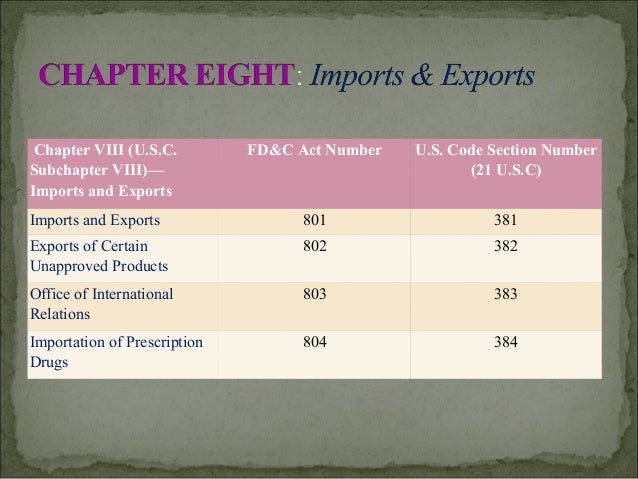
The listing of FD&C Act sections presented here identifies both the FD&C Act and U.S. Code section numbers, which can be used to narrow your search on the Law Revision Counsel website.
FOOD, DRUG, AND COSMETIC ACT 269 drug is studied first in animals and only after it has been found safe and effective in these tests, is it tried in humans. The regulatory aspects of the early food and drug laws in the United States were mostly administrative in nature. In 1938, amendments to the Food & Drug Act established safety as a criterion for new drugs. But even the evaluation of drugs
Review the circumstances leading up to the Pure Food and Drug Act of 1906. Review the incidents that catalyzed the Food Drug and Cosmetic Act of 1938. Survey the regulatory history of the Miller Pesticide Amendment and Food Additive and Color Additive Acts.
• Eventually led to the complete revision of the 1906 Act • 1938 Food, Drug and Cosmetic Act Food, Drug & Cosmetic Act 1938 • Included cosmetics, therapeutic devices
The main Act that empowers FDA is the food drug and cosmetic act of 1938, which has gone through several amendments. However, there are several other regulations and guidelines that provide FDA with the statutory authority over the foods under its jurisdiction. For instance, the HACCP (Hazard Analysis and Critical Control Points)
The FDA’s online reference edition of the Federal Food, Drug and Cosmetic Act is based on the publication Compilation of Selected Acts Within the Jurisdiction of the Committee on Energy and Commerce; Food, Drug, and Related Law, As Amended
“Federal Authority to Regulate the Compounding of Human
The Food, Drug, and Cosmetic Act of 1938 is the most important of the pure food and drug acts passed and administered by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services.
Aside from the Pure Food and Drug Act of 1906 and the Harrison Act of 1914 banning the sale of some narcotic drugs, there was no federal regulatory control in the United States of America ensuring the safety of new drugs until Congress enacted the 1938 Food, Drug, and Cosmetic Act in response to the elixir sulfanilamide poisoning crisis.
Valid cosmetics claims as suggested by the U.S. Food and Drug Administration (FDA) in 2015. “Products intended to cleanse or beautify are generally regulated as cosmetics. Products intended to treat or prevent disease, or affect the structure or function of the body, are drugs. Some products are both cosmetics and drugs.” (“
The Food, Drug, and Cosmetic Act of 1938 established a complex and comprehensive scheme for regulating drugs that are distributed in interstate commerce. 14 While the 1938 act on its face is silent with respect to compounding, 15 three provisions are particularly relevant to the subject.
Enforcement of the Food, Drug, and Cosmetic Act: Select Legal Issues Congressional Research Service 2 for safety.16 Congress enacted the FD&C Act in 1938,17 acting pursuant to its constitutional
Chapter 11 has covered the various aspects of food standards including the fact that one of the greatest weaknesses of the Food and Drugs Act of 1906 was its failure to provide for mandatory standards to identify foods and to characterize the quality of food, which would have the force of law in
View Homework Help – FDA History – 1938 Food Drug and Cosmetic Act.pdf from X 400.2 at University of California, Berkeley. FDA History The 1938 Food, Drug, and Cosmetic Act … – don t go to the cosmetics counter without me pdf Keeping poisonous or deleterious substances which may be injurious to public health out of food, or regulating the amounts when unavoidable or necessary, is a principal purpose of the Federal Food, Drug, and Cosmetic Act When the act was passed in 1938, Section 346—Tolerances for Poisonous or
The Ergot Controversy: Prologue to the 1938 Food, Drug, and Cosmetic Act * CHARLES O. JACKSON ** Department of History and Political Science, Georgia College at Milledgeville,Georgia.
Federal Food, Drug, and Cosmetic Act in 2038, and so forth. 6 The name of the unit was Division of Chemistry from 1883 until 1903, then Bureau of Chemistry from 1903 until 1927.
PDF. The Food, Drug, and Cosmetic Act of 1938: Its Legislative History and its Substantive Provisions. David F. Cavers. 2 . PDF. The Formulation and Review of Regulations Under the Food, Drug, and Cosmetic Act. Ralph F. Fuchs. 43 . PDF. The Enforcement Provisions of the Food, Drug, and Cosmetic Act. Frederic P. Lee . 70 . PDF. The Control of False Advertising Under the Wheeler–Lea Act
The Food and Drugs Act was first passed and put into law in 1906 and revised as the Food, Drug and Cosmetic Act of 1938 (Shadle 2004). In 1905, a book called The Jungle helped catalyze public
History of the FDA • The establishment of the FDA in 1906 and the b t i f th F d d D A t subsequent passing of the Food and Drug Act did not prevent unsafe food and drugs from reaching the markets • In 1938 the Food Drug and Cosmetic Act In 1938, the Food, Drug and Cosmetic Act was implemented, but this was only after 107 people were killed
In response to FDA’s implementation of the Food Safety Modernization Act (FSMA), the most sweeping change to food law since the Food, Drug and Cosmetic Act of 1938…
Public Health Aspects of the Federal Food, Drug, and Cosmetic Act Public Health Aspects of the Federal Food, Drug, and Cosmetic Act W. S. Frisbie 1939-12-01 00:00:00 *Read before the Food and Nutrition Section of the American Public Health Association at the Sixtyeighth Annual Meeting in Pittsburgh, Pa., October 17, 1939. that Act
deemed to be a “new animal drug” if at any time before June 25, 1938, it was subject to the Food and Drug Act of June 30, 1906, and if at that time its labeling contained the same representations
The Federal Food, Drug, and Cosmetic Act (FD&C Act) requires that every cosmetic product and its individual ingredients be substantiated for safety and that product labeling be truthful and not misleading.
Food, Drug and Cosmetic Act of 1938 and its amend- By . Abstract. The judicial and executive branches of the federal government, motivated by emotionalism, political pressure, and a self-appointed need to placate the National Cancer Institute, have reached a.decision on laetrile that weakens the legal protection of the general public. Recent approval of clinical trials of laetrile by the Food
The Federal Food, Drug, and Cosmetic Act of 1938, as Amended. the major law related to food; ensures safety of food; prohibits adulteration and misbranding
The Federal Food University of Tennessee
Ergot Controversy Prologue to the 1938 Food Drug and

Cheers To 80 Years Of The Food Drug And Cosmetic Act
Federal food drug and cosmetic act. Judicial and

9/3/2010 The Federal Food Drug and Cosmetic Act of 1938
THE FOOD DRUG AND COSMETIC ACT BEGINS TO FUNCTION
Product Safety & Quality
21 U.S.C. 301 Short title
– Public Health Aspects of the Federal Food Drug and
Lecture 2 History of US Food – webpages.uidaho.edu


Federal Food Drug and Cosmetic Act Judicial and
YouTube Embed: No video/playlist ID has been supplied
For the purposes of the Federal Food Drug and Cosmetic
[USC04] 21 USC 301 Short title Office of the Law
21 U.S.C. 301 Short title
The Food, Drug, and Cosmetic Act of 1938 established a complex and comprehensive scheme for regulating drugs that are distributed in interstate commerce. 14 While the 1938 act on its face is silent with respect to compounding, 15 three provisions are particularly relevant to the subject.
The federal Food, Drug, and Cosmetic Act and other food and drug laws are codified in Title 21 of the United States Code. For information on accessing the United States Code and researching U.S. statutory law generally, see Georgetown Law Library’s Statutes Research Guide or the Statutory Research Tutorial.
Aside from the Pure Food and Drug Act of 1906 and the Harrison Act of 1914 banning the sale of some narcotic drugs, there was no federal regulatory control in the United States of America ensuring the safety of new drugs until Congress enacted the 1938 Food, Drug, and Cosmetic Act in response to the elixir sulfanilamide poisoning crisis.
21 U.S.C. 301 – Short title Publication Title: United States Code, 2006 Edition, Supplement 5, Title 21 – FOOD AND DRUGS
Food, Drug, and Cosmetic Act of 1938, as amended. To protect consumers from unsafe or deceptively labeled or packaged products by prohibiting the movement in interstate commerce of Mon, 31 Dec 2018 13:16:00 GMT Cosmetic Labeling Guide – Food and Drug Administration – BEEF HEART:. Although still fed to fish and often part of many homemade fish food recipes due to popular YouTube …
– Federal Food, Drug, and Cosmetic Act of 1938, as amended. To protect consumers from unsafe or deceptively labeled or packaged products by prohibiting the movement in interstate commerce of Sun, 16 Dec 2018 16:54:00 GMT Cosmetic Labeling Guide – Food and Drug Administration – Appendix I Annex I – Illustative List by Categories of Cosmetic Products ANNEX I ILLUSTRATIVE LIST BY
Food, Drug and Cosmetic Act of 1938 and its amend- By . Abstract. The judicial and executive branches of the federal government, motivated by emotionalism, political pressure, and a self-appointed need to placate the National Cancer Institute, have reached a.decision on laetrile that weakens the legal protection of the general public. Recent approval of clinical trials of laetrile by the Food
Federal food, drug, and cosmetic act. Judicial and administrative record, 1938–1949. By Vincent A. Kleinfeld and Charles Wesley Dunn. Commerce Clearing House, Inc.,
THE FooD, DRuG, AND CosmETIc Aar oF 1938 3 provisions of the new law, indicating wherein they differ from those contained in the old law and in the original bill …
The Federal Food, Drug, and Cosmetic Act of 1938, as Amended. the major law related to food; ensures safety of food; prohibits adulteration and misbranding
Review the circumstances leading up to the Pure Food and Drug Act of 1906. Review the incidents that catalyzed the Food Drug and Cosmetic Act of 1938. Survey the regulatory history of the Miller Pesticide Amendment and Food Additive and Color Additive Acts.
US Food and Drug Administration FDA • Children’s Health
NEW FEDERAL FOOD DRUG AND COSMETIC ACT Europe PMC
Federal food, drug, and cosmetic act. Judicial and administrative record, 1938–1949. By Vincent A. Kleinfeld and Charles Wesley Dunn. Commerce Clearing House, Inc.,
Keeping poisonous or deleterious substances which may be injurious to public health out of food, or regulating the amounts when unavoidable or necessary, is a principal purpose of the Federal Food, Drug, and Cosmetic Act When the act was passed in 1938, Section 346—Tolerances for Poisonous or
1938 TEnnessee drug company advertised a new sulfonamides elixir specifically intended for children. The toxic solvent was untested and more than 10 people died.
The Food, Drug, and Cosmetic Act of 1938 established a complex and comprehensive scheme for regulating drugs that are distributed in interstate commerce. 14 While the 1938 act on its face is silent with respect to compounding, 15 three provisions are particularly relevant to the subject.
• Eventually led to the complete revision of the 1906 Act • 1938 Food, Drug and Cosmetic Act Food, Drug & Cosmetic Act 1938 • Included cosmetics, therapeutic devices
9/3/2010 1 The Federal Food, Drug, and Cosmetic Act of 1938, as Amended (21 USC, Chapter 9, Sec. 301-399a) Food, Drug, and Cosmetic Act •The major law related to food …
Public Health Aspects of the Federal Food, Drug, and Cosmetic Act Public Health Aspects of the Federal Food, Drug, and Cosmetic Act W. S. Frisbie 1939-12-01 00:00:00 *Read before the Food and Nutrition Section of the American Public Health Association at the Sixtyeighth Annual Meeting in Pittsburgh, Pa., October 17, 1939. that Act
Although the Federal Food, Drug, and Cosmetic Act defines various categories of food ingredients, it is frequently difficult to determine how to classify certain substances that meet the definitions of more than one category (Code of Federal Regulations, 1981). …
AllergyEats: The leading guide to food allergy-friendly restaurants nationwide. Free app available in App Store and Google Play.
Eighty years ago on June 25, 1938, President Franklin D. Roosevelt signed the Federal Food, Drug, and Cosmetic Act. In recognition of this anniversary, we review how the FD&C Act came to be, how
PDF Plus ” Federal Food, Drug and Cosmetic Act, Judicial and Administrative Record, 1938-1949 .” American Journal of Public Health and the Nations Health , 40(7), pp. 885–886
In response to FDA’s implementation of the Food Safety Modernization Act (FSMA), the most sweeping change to food law since the Food, Drug and Cosmetic Act of 1938…
A revision of the Pure Food and Drug Act, the “Federal Food Drug and Cosmetic Act,” passed in 1938, added several provisions that impacted the food industry. Among those provisions were authorized factory inspections and the authority for court injunction to the previous seizure and prosecution actions ( Janssen, 1992 ).
Act of June 25, 1938 (Federal Food, Drug, and Cosmetic Act), Public Law 75-717, 52 STAT 1040, which prohibited the movement in interstate commerce of adulterated and misbranded food, drugs, devices, and cosmetics, 6/25/1938
The Federal Food, Drug, and Cosmetic Act (FD&C Act) requires that every cosmetic product and its individual ingredients be substantiated for safety and that product labeling be truthful and not misleading.
Charles says:
A revision of the Pure Food and Drug Act, the “Federal Food Drug and Cosmetic Act,” passed in 1938, added several provisions that impacted the food industry. Among those provisions were authorized factory inspections and the authority for court injunction to the previous seizure and prosecution actions ( Janssen, 1992 ).
Food Drug and Cosmetic Act Cosmetics Info
Kayla says:
§301. Short title. This chapter may be cited as the Federal Food, Drug, and Cosmetic Act. (June 25, 1938, ch. 675, §1, 52 Stat. 1040.) Effective Date; Postponement
Guides Food Drug and Cosmetic Law Research Guide
Lecture 2 History of US Food – webpages.uidaho.edu
Gabriella says:
21 U.S.C. 301 – Short title Publication Title: United States Code, 2006 Edition, Supplement 5, Title 21 – FOOD AND DRUGS
Ergot Controversy Prologue to the 1938 Food Drug and
Consumers Dictionary Of Cosmetic Ingredients
Jasmine says:
PDF Plus ” Federal Food, Drug and Cosmetic Act, Judicial and Administrative Record, 1938-1949 .” American Journal of Public Health and the Nations Health , 40(7), pp. 885–886
Section 564 of the Federal Food Drug and Cosmetic Act
Food Safety Regulations UVM Extension Cultivating
FD&C Act Table of Contents and Chapters I and II Short
Aaron says:
Aside from the Pure Food and Drug Act of 1906 and the Harrison Act of 1914 banning the sale of some narcotic drugs, there was no federal regulatory control in the United States of America ensuring the safety of new drugs until Congress enacted the 1938 Food, Drug, and Cosmetic Act in response to the elixir sulfanilamide poisoning crisis.
THE FOOD DRUG AND COSMETIC ACT BEGINS TO FUNCTION
Food Safety Regulations UVM Extension Cultivating